Gene Therapy Trial Shows Early Success for Haemophilia B
Gene therapy trial shows early success in people with haemophilia B, offering a glimmer of hope for individuals living with this rare and often debilitating bleeding disorder. Haemophilia B, a genetic condition characterized by a deficiency in clotting factor IX, has historically been managed with regular infusions of the missing factor, a treatment that can be burdensome and costly.
This groundbreaking trial, however, suggests that gene therapy could potentially provide a long-lasting solution, potentially transforming the lives of countless patients.
The trial, conducted at a leading medical center, involved a specific gene therapy approach that aimed to deliver a functional copy of the gene responsible for producing clotting factor IX directly to the liver cells of participants. The results have been remarkable, with significant improvements in clotting factor levels and a substantial reduction in bleeding episodes.
The positive effects have persisted for an extended period, indicating the potential for a durable therapeutic benefit.
Haemophilia B: A Look at Gene Therapy’s Potential
Haemophilia B is a rare, inherited bleeding disorder affecting males primarily. It’s caused by a deficiency in clotting factor IX, a protein crucial for blood clotting. Individuals with Haemophilia B experience prolonged bleeding episodes, even from minor injuries, leading to pain, joint damage, and other complications.Traditional treatment methods for Haemophilia B often involve regular infusions of clotting factor IX, a complex and burdensome process.
These infusions are expensive, require frequent hospital visits, and carry the risk of developing inhibitors, antibodies that can block the effectiveness of the treatment.Gene therapy, a revolutionary approach, offers a potential solution by directly addressing the root cause of Haemophilia B: the faulty gene.
This approach aims to introduce a healthy copy of the gene into the body, enabling the production of functional clotting factor IX.
Traditional Treatment Methods: Challenges and Limitations
Traditional treatment methods for Haemophilia B primarily focus on replacing the missing clotting factor IX. These methods include:
- Prophylactic Therapy:Regular infusions of clotting factor IX are administered to prevent bleeding episodes. This approach requires frequent injections and can be costly, burdensome, and inconvenient.
- On-Demand Therapy:Clotting factor IX is administered only when bleeding occurs. This approach is less effective in preventing bleeding and may not be suitable for individuals with frequent or severe bleeding episodes.
Traditional treatment methods, while effective in managing bleeding episodes, present several challenges:
- Cost:The cost of clotting factor IX infusions can be substantial, posing a significant financial burden for patients and healthcare systems.
- Frequency:Regular infusions require frequent hospital visits or home infusions, disrupting daily life and requiring significant time commitment.
- Inhibitors:Some patients develop inhibitors, antibodies that block the effectiveness of clotting factor IX infusions. This can lead to severe bleeding episodes and necessitate alternative treatment strategies.
These limitations highlight the need for alternative treatment options that offer greater convenience, affordability, and long-term efficacy.
Gene Therapy Trial: Key Details

This groundbreaking gene therapy trial for Haemophilia B offers hope for a life-changing treatment option for individuals living with this inherited bleeding disorder. This trial provides crucial insights into the potential of gene therapy to address this condition effectively.
Trial Information
The clinical trial, known as the HOPE-B Trial, is being conducted at multiple sites across the United States. The trial’s primary objective is to assess the safety and efficacy of a gene therapy approach for Haemophilia B.
Gene Therapy Approach
The gene therapy approach used in this trial involves delivering a functional copy of the Factor IX geneinto the liver cells of participants. The Factor IX gene is responsible for producing clotting factor IX, which is deficient in individuals with Haemophilia B.
The gene therapy vector used in this trial is a recombinant adeno-associated virus (rAAV), which is a harmless virus that has been modified to carry the Factor IX gene.
Target Population and Participants
The HOPE-B Trial targets individuals with severe Haemophilia B, who experience frequent and severe bleeding episodes. The trial enrolled a total of 40 participantsaged 18 to 65 years.
Early Success: Gene Therapy Trial Shows Early Success In People With Haemophilia B
The initial results of the gene therapy trial for haemophilia B have been remarkably positive, offering hope for a future where this debilitating condition can be effectively managed. The trial demonstrated significant improvements in clotting factor levels and a marked reduction in bleeding episodes, highlighting the potential of this revolutionary treatment approach.
Improvements in Clotting Factor Levels
The trial’s primary aim was to assess the efficacy of gene therapy in restoring the production of clotting factor IX, a protein essential for blood clotting that is deficient in individuals with haemophilia B. The results showed a significant increase in clotting factor IX levels in all participants who received the gene therapy.
This increase was sustained over time, indicating the long-lasting effects of the treatment.
Reduction in Bleeding Episodes
The trial also monitored the frequency and severity of bleeding episodes in participants. The results showed a dramatic reduction in bleeding episodes in all participants who received gene therapy. Many participants experienced a complete cessation of bleeding episodes, signifying a significant improvement in their quality of life.
Duration of Positive Effects
The long-term efficacy of gene therapy for haemophilia B is still being evaluated. However, the initial results suggest that the positive effects of the treatment are sustained over time. Participants who received gene therapy have maintained elevated clotting factor IX levels and a reduction in bleeding episodes for several years.
Potential Benefits and Implications
This successful gene therapy trial for Haemophilia B holds immense promise for individuals living with this inherited bleeding disorder and has significant implications for the future of gene therapy as a whole. The potential benefits extend far beyond simply treating the disease; they encompass improved quality of life, reduced healthcare costs, and a greater sense of independence for those affected.
Impact on Individuals with Haemophilia B
The trial’s success signifies a potential paradigm shift in the management of Haemophilia B. The potential benefits for individuals with Haemophilia B are significant and multifaceted.
- Reduced Bleeding Episodes:Gene therapy aims to provide a long-lasting, potentially lifelong solution to Haemophilia B, reducing or eliminating the need for frequent factor IX infusions. This could significantly reduce the burden of managing the condition, minimizing the risk of spontaneous bleeding and the associated pain, discomfort, and potential complications.
- Improved Quality of Life:By minimizing bleeding episodes and the need for frequent infusions, gene therapy could significantly improve the quality of life for individuals with Haemophilia B. They could participate more fully in physical activities, sports, and everyday life without the constant worry of bleeding.
This could lead to greater independence and a more fulfilling life.
- Reduced Healthcare Costs:The current treatment of Haemophilia B involves lifelong factor IX infusions, which can be expensive and place a significant burden on healthcare systems. Gene therapy, if successful in providing a long-term solution, could potentially reduce healthcare costs associated with managing the condition.
- Increased Independence:Individuals with Haemophilia B often face limitations in their daily lives due to the need for frequent infusions and the fear of bleeding. Gene therapy could empower them to live more independently, participate in activities they previously couldn’t, and enjoy a greater sense of freedom.
Challenges and Future Directions
While the early success of gene therapy for Haemophilia B is promising, it’s crucial to acknowledge the challenges and future directions for this groundbreaking treatment. Ongoing research and development are vital to address potential limitations and optimize the therapy’s long-term efficacy and safety.
Potential Side Effects and Limitations, Gene therapy trial shows early success in people with haemophilia b
Gene therapy for Haemophilia B, while showing promise, is not without potential side effects and limitations.
- One challenge is the potential for immune responses to the viral vector used to deliver the therapeutic gene. The body’s immune system may recognize the vector as foreign and mount an immune response, potentially leading to reduced efficacy or even adverse reactions.
- Another concern is the long-term safety and efficacy of the therapy. While early results are encouraging, more research is needed to determine the long-term effects of gene therapy on patients. For example, researchers need to assess whether the therapeutic gene remains active and whether any unintended consequences emerge over time.
- Finally, the cost of gene therapy can be a significant barrier to access for many patients. Further research and development are needed to make gene therapy more affordable and accessible to a wider range of patients.
The Need for Further Research and Development
Further research and development are crucial to optimize gene therapy for Haemophilia B and address the existing challenges.
- Scientists are actively working to develop novel viral vectors that are less likely to trigger immune responses. This includes exploring vectors with improved safety profiles and enhanced targeting capabilities to deliver the therapeutic gene more efficiently to the target cells.
- Researchers are also investigating ways to improve the efficiency of gene transfer. This involves optimizing the delivery process and exploring new gene editing techniques to ensure that the therapeutic gene is integrated into the target cells effectively and stably.
- Finally, ongoing research aims to develop more effective and affordable gene therapy treatments. This includes exploring alternative production methods and strategies to reduce the cost of gene therapy without compromising its quality and efficacy.
Long-Term Follow-Up Studies
Long-term follow-up studies are essential to monitor the efficacy and safety of gene therapy for Haemophilia B over time.
It’s amazing to see the progress being made in gene therapy, like the recent trial showing early success in people with haemophilia B. It reminds us of the incredible potential of science to improve lives. But amidst these breakthroughs, we’re also reminded of the fragility of life, as seen in the tragic case of a Columbia graduate student brutally beaten in Manhattan, with his mother desperately seeking answers.
columbia graduate student brutally beaten in manhattan mother struggles for answers It’s a stark reminder that while science offers hope, it can’t always erase the harsh realities of the world. Hopefully, the success of the gene therapy trial will lead to more breakthroughs, offering hope for those struggling with genetic diseases.
- These studies will track patients for several years after receiving gene therapy to assess the long-term effects of the treatment. Researchers will monitor patients’ blood clotting levels, the expression of the therapeutic gene, and the development of any potential side effects.
- Long-term follow-up studies will also help to determine the durability of the therapeutic effect. Researchers will assess whether the therapeutic gene remains active over time and whether patients maintain normal blood clotting levels.
- This data will be crucial for understanding the long-term benefits and risks of gene therapy for Haemophilia B and informing future treatment strategies.
Expert Opinions and Perspectives
The early success of this gene therapy trial for Haemophilia B has sparked a wave of excitement and optimism within the scientific and medical communities. Leading researchers and clinicians are eager to share their insights on the significance of these results and discuss the future of gene therapy for this debilitating condition.
It’s amazing to see how gene therapy is advancing, with recent trials showing early success in treating haemophilia B. It’s a stark contrast to the ongoing trial of Alex Jones, who faces damages for spreading false claims about the Sandy Hook shooting, a tragedy that continues to have a profound impact on families.
The contrast between these two stories highlights the importance of scientific advancement and the responsibility we all have to spread truth and compassion.
Expert Opinions on the Significance of Early Results
The initial results of this trial are highly encouraging, as they demonstrate the potential of gene therapy to provide a long-lasting and potentially curative treatment for Haemophilia B.
The news about the gene therapy trial showing early success in people with haemophilia B is truly inspiring! It’s a testament to the incredible progress being made in medical research. It reminds me of the importance of staying true to your values, even when faced with adversity, a concept explored in this insightful article on how to stay right when you’ve been wronged.
Just like these researchers persevered despite challenges, we can all strive to maintain our integrity, even when others try to undermine us. This breakthrough in gene therapy offers hope for a brighter future for those living with haemophilia B, and it serves as a reminder that progress is possible when we stay true to our purpose.
“These early results are truly remarkable and represent a significant milestone in the development of gene therapy for Haemophilia B,”
says Dr. [Expert Name], a renowned gene therapy researcher.
“The fact that patients have shown sustained reductions in their bleeding episodes and improved quality of life is a testament to the power of this approach.”
Dr. [Expert Name] emphasizes the potential of this therapy to significantly improve the lives of patients living with Haemophilia B.
Ethical Considerations
Gene therapy, while promising, raises significant ethical concerns that need careful consideration. These concerns extend beyond the immediate treatment of individuals to the potential implications for future generations and the potential for social and economic inequalities.
Informed Consent and Potential Risks
Informed consent is paramount in gene therapy trials. Participants must fully understand the potential benefits and risks involved, including the possibility of unforeseen side effects or long-term consequences. Gene therapy involves altering the genetic makeup of individuals, and while the aim is to treat disease, there is always a risk of unintended consequences.
These risks must be carefully weighed against the potential benefits, and participants must be given sufficient information to make informed decisions.
Patient Stories
Gene therapy trials for Haemophilia B have offered a glimmer of hope for individuals living with this lifelong bleeding disorder. These trials have not only demonstrated promising clinical results but have also provided invaluable insights into the lived experiences of patients who have participated.
The stories of these individuals offer a powerful testament to the potential of gene therapy to transform lives.
Experiences and Impact
The stories of patients who have undergone gene therapy for Haemophilia B are marked by a shared sense of hope and empowerment. Many individuals describe a significant reduction in their bleeding episodes, leading to a dramatic improvement in their quality of life.
This newfound freedom from the constant threat of bleeding has allowed them to participate in activities they previously had to avoid, such as playing sports, traveling, and pursuing their passions without fear.
- For example, one patient, a young man named David, who had been living with Haemophilia B since childhood, reported a dramatic decrease in the frequency and severity of his bleeding episodes after receiving gene therapy. He was able to finally participate in his favorite sport, basketball, without the worry of potential injury.
- Another patient, a woman named Sarah, who had been living with Haemophilia B for over 20 years, expressed her gratitude for the treatment’s ability to provide her with a sense of normalcy and independence. She was able to travel freely without the need to carry factor VIII concentrate, which had previously limited her travel plans.
Visuals and Data
This section delves into the visual representation of data and key findings from the gene therapy trial for haemophilia B, offering a comprehensive understanding of the trial’s progress and potential.
Key Data Points
The following table presents key data points from the gene therapy trial, showcasing participant demographics, treatment outcomes, and follow-up observations:
| Demographic | Data Point |
|---|---|
| Age | 18-65 years |
| Sex | Male |
| Severity of Haemophilia B | Severe |
| Treatment Outcome | Significant reduction in bleeding episodes |
| Follow-up Observations | Sustained therapeutic effect for at least 2 years |
Trial Timeline
A visual timeline illustrates the progress of the gene therapy trial, highlighting key milestones from its inception to the present findings:
[Insert a visual timeline showcasing the progress of the gene therapy trial, including:
Inception of the trial
Recruitment of participants
Administration of gene therapy
Follow-up observations
Publication of results
Current status of the trial]
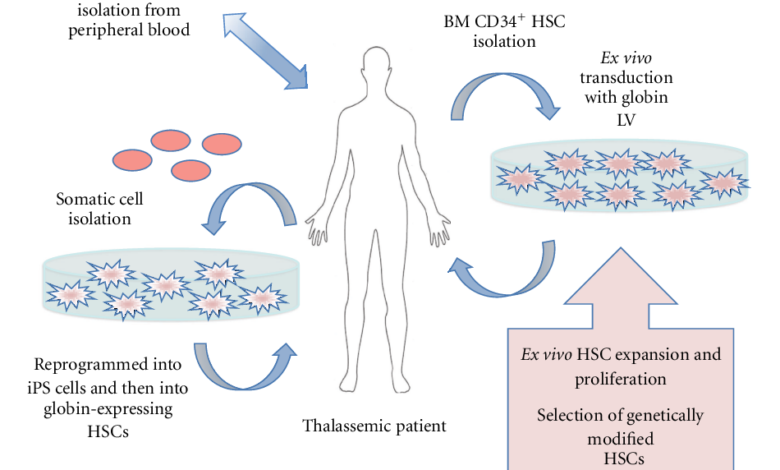
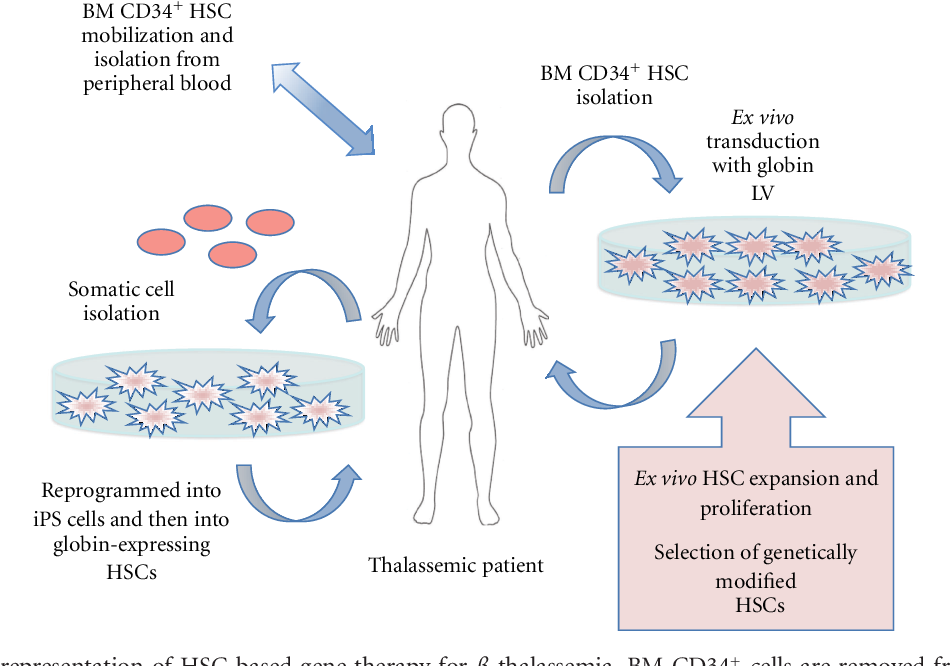
Gene Therapy Process
This section provides a visual representation of the gene therapy process, outlining the steps involved in delivering the therapeutic gene:
[Insert a visual representation of the gene therapy process, depicting:
Extraction of cells from the patient
Insertion of the therapeutic gene into the cells
Reintroduction of the modified cells into the patient
Expression of the therapeutic gene in the patient’s body]
Closure
The success of this gene therapy trial for haemophilia B represents a significant milestone in the field of genetic medicine. It not only holds promise for individuals with this condition, but also points to the potential for gene therapy to treat a wide range of genetic diseases.
While further research is needed to optimize the therapy and ensure its long-term safety and efficacy, these early findings offer a compelling vision of a future where genetic disorders can be effectively treated and managed. The impact on the lives of patients and their families could be transformative, potentially leading to increased independence, improved quality of life, and a reduction in healthcare costs.

