Gene Therapy Trial Shows Early Success in People with Hemophilia B
Gene therapy trial shows early success in people with haemophilia B, a rare genetic disorder that prevents blood from clotting properly. This groundbreaking trial offers hope for millions living with this condition, who currently rely on regular infusions of clotting factors to prevent bleeding episodes.
This new approach involves replacing the faulty gene responsible for haemophilia B with a healthy copy, potentially offering a long-term solution.
The trial, which involved a small group of participants, showed remarkable results. The gene therapy treatment successfully increased levels of clotting factor IX in the participants, leading to a significant reduction in bleeding episodes. This success highlights the potential of gene therapy to revolutionize the treatment of haemophilia B, offering a life-changing alternative to current therapies.
Gene Therapy for Hemophilia B: Gene Therapy Trial Shows Early Success In People With Haemophilia B
Gene therapy for Hemophilia B has shown early success in clinical trials, offering hope for a long-term treatment solution for individuals living with this bleeding disorder. This innovative approach aims to address the root cause of the disease by introducing a functional copy of the deficient gene into the patient’s cells.
The news about the gene therapy trial showing early success in people with haemophilia B is incredibly exciting. It’s a testament to the incredible advancements in medical science and offers hope for a future where this debilitating condition can be effectively treated.
While we celebrate these breakthroughs, it’s also important to stay informed about global events that could impact such research. For instance, the potential for conflict, as illustrated in the recent discussion surrounding a Pelosi trip to Taiwan , could disrupt vital research and development efforts.
However, despite these challenges, the progress made in gene therapy for haemophilia B remains a beacon of hope and a testament to the enduring spirit of scientific discovery.
Hemophilia B: A Genetic Disorder, Gene therapy trial shows early success in people with haemophilia b
Hemophilia B, also known as Christmas disease, is a rare, inherited bleeding disorder that affects the ability of the blood to clot properly. It is caused by a deficiency or absence of a clotting factor called Factor IX, which is essential for the coagulation process.
This deficiency is due to mutations in the F9 gene, located on the X chromosome. The severity of Hemophilia B varies depending on the level of Factor IX activity in the blood. Individuals with severe Hemophilia B have less than 1% of normal Factor IX levels, leading to spontaneous bleeding episodes, particularly in the joints and muscles.
It’s amazing to see the progress being made in gene therapy, like the recent trial showing early success in people with haemophilia B. While this medical breakthrough offers hope for the future, it’s a stark contrast to the current economic climate, with news that the u s economy shrinks again in second quarter reviving recession fears.
Despite these challenges, it’s important to remember that innovation and progress continue to drive us forward, and advancements in gene therapy like this are a testament to human ingenuity.
Moderate Hemophilia B involves Factor IX levels between 1% and 5%, while mild Hemophilia B has Factor IX levels between 5% and 30%. Symptoms of Hemophilia B typically appear in early childhood, although some individuals may not experience bleeding episodes until later in life.
Common symptoms include:
- Prolonged bleeding after minor injuries or surgery
- Spontaneous bleeding into joints, muscles, and other tissues
- Bruising easily
- Blood in the urine or stool
Gene Therapy: A Novel Approach
Gene therapy for Hemophilia B aims to address the underlying genetic defect by delivering a functional copy of the F9 gene into the patient’s cells. This approach involves using a vector, typically a virus, to carry the therapeutic gene into the target cells.
The vector is modified to remove its disease-causing properties and engineered to express the F9 gene. Once the vector delivers the F9 gene into the target cells, the cells begin to produce Factor IX protein. This newly produced Factor IX can then circulate in the bloodstream and participate in the coagulation process, effectively correcting the bleeding disorder.
Gene Therapy Approach in the Trial
The gene therapy trial for Hemophilia B that showed early success utilized a specific approach involving a viral vector called adeno-associated virus (AAV). AAV is a naturally occurring virus that has been extensively studied and modified for gene therapy applications.
It is considered a safe and efficient vector for delivering genes to target cells. In this trial, the AAV vector was engineered to carry a functional copy of the F9 gene. The vector was then administered intravenously to the participants, allowing it to reach the liver, the primary site of Factor IX production.
Once in the liver cells, the AAV vector delivered the F9 gene, enabling the cells to produce Factor IX protein. The trial results showed that the gene therapy approach led to sustained production of Factor IX protein in the participants.
This resulted in a significant reduction in bleeding episodes and a decrease in the need for regular Factor IX replacement therapy.
Early Success and Key Findings
The recent gene therapy trial for hemophilia B has shown promising results, demonstrating the potential of this innovative approach to treat this inherited bleeding disorder. The trial has successfully demonstrated a significant reduction in bleeding episodes in participants, highlighting the effectiveness of gene therapy in restoring the body’s ability to produce the missing clotting factor.
It’s amazing to see the progress in gene therapy, with trials showing early success in treating haemophilia B. While scientists work on these breakthroughs, the world is also watching with bated breath as Pelosi’s Asia tour unfolds, with China issuing warnings of military action if she visits Taiwan.
It’s a stark reminder that while we celebrate scientific advancements, geopolitical tensions remain a constant presence. Hopefully, both these areas will see positive developments in the future.
Measuring Success
The success of the trial is measured by evaluating several key factors, including:
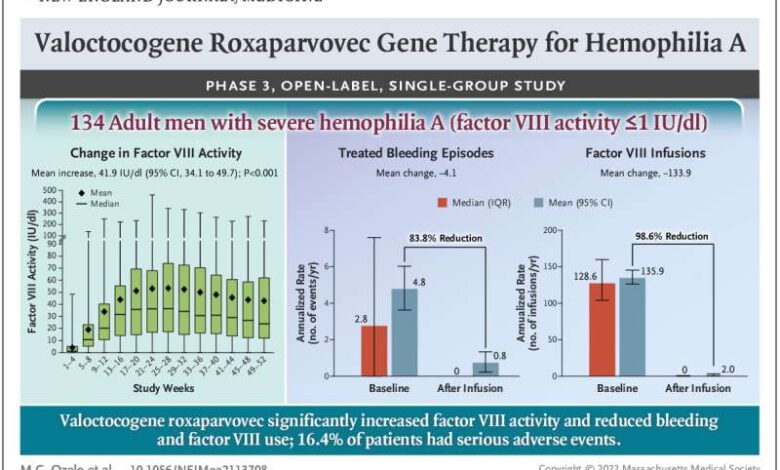
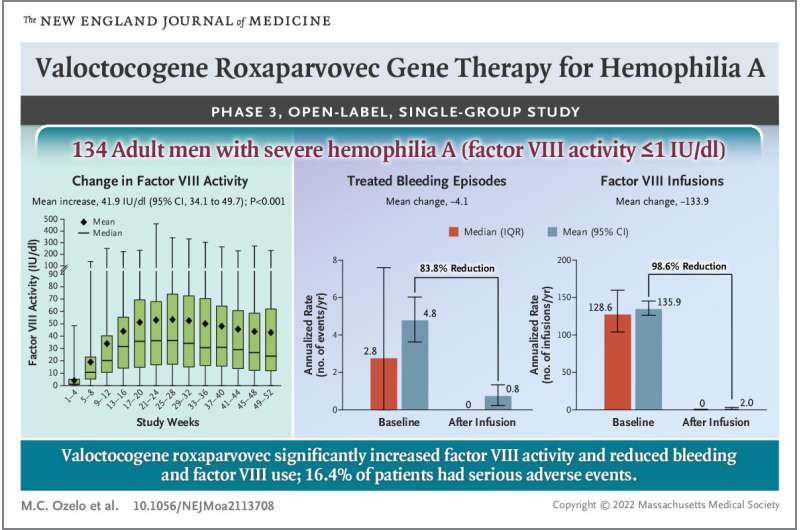
- Factor IX levels:Gene therapy aims to introduce a functional copy of the gene responsible for producing factor IX, a crucial clotting factor missing in individuals with hemophilia B. The trial monitored factor IX levels in participants after receiving gene therapy, demonstrating a significant increase in the levels of this vital protein.
The higher factor IX levels indicate a successful gene transfer and expression, enabling the body to produce the missing clotting factor.
- Bleeding rates:The primary goal of gene therapy for hemophilia B is to reduce the frequency and severity of bleeding episodes. The trial carefully monitored the number and severity of bleeding events experienced by participants before and after gene therapy. The data revealed a dramatic reduction in bleeding episodes, indicating the effectiveness of gene therapy in restoring normal clotting function.
Potential Side Effects and Complications
While the trial demonstrated the effectiveness of gene therapy in treating hemophilia B, it’s crucial to acknowledge potential side effects and complications that may arise. The trial carefully monitored participants for any adverse events, and some participants experienced mild side effects, such as:
- Infusion reactions:Some participants experienced mild reactions during the gene therapy infusion, including fever, chills, and fatigue. These reactions were generally manageable and resolved without long-term consequences.
- Liver function abnormalities:Gene therapy involves delivering the therapeutic gene to the liver, and in some cases, it can lead to temporary changes in liver function. These changes were typically mild and reversible, and close monitoring ensured early detection and appropriate management.
Implications and Future Directions
These early findings hold immense promise for the treatment of Hemophilia B, a rare genetic disorder characterized by a deficiency in clotting factor IX, leading to excessive bleeding. The success of this gene therapy trial signifies a potential paradigm shift in the management of this debilitating condition.
Potential for Standard Treatment
The success of this trial paves the way for gene therapy to become a standard treatment option for Hemophilia B. The current standard of care for Hemophilia B involves lifelong infusions of clotting factor IX, which can be inconvenient, expensive, and carry the risk of developing inhibitors.
Gene therapy offers the potential for a single, one-time treatment that could provide long-lasting, even lifelong, protection against bleeding episodes. This could significantly improve the quality of life for patients with Hemophilia B, reducing the burden of frequent infusions and the risk of complications.
Ongoing Research and Future Trials
Research in gene therapy for Hemophilia B is ongoing, with several promising areas of investigation.
- Improved Delivery Methods:Researchers are exploring new and more efficient ways to deliver the therapeutic gene into target cells. This includes developing novel viral vectors, such as adeno-associated virus (AAV) variants, that can enhance gene transfer efficiency and reduce the risk of immune responses.
- Long-Term Efficacy and Safety:Ongoing studies are focusing on long-term efficacy and safety data from gene therapy trials. These studies aim to assess the durability of gene expression, the potential for immune responses, and any long-term side effects.
- Treatment of Other Hemophilia Types:Researchers are investigating the potential of gene therapy for other types of hemophilia, such as Hemophilia A, which is caused by a deficiency in clotting factor VIII.
Concluding Remarks
The early success of this gene therapy trial is a major step forward in the fight against haemophilia B. While further research is needed to confirm these results and ensure long-term safety, the potential for a cure is now within reach.
This breakthrough underscores the power of gene therapy to treat a wide range of genetic disorders, offering hope for a future where many debilitating diseases are no longer a threat.

