US Licenses Key COVID Vaccine Technology to WHO
U s licenses key covid vaccine technology to who so other countries can develop shots – US Licenses Key COVID Vaccine Technology to WHO so other countries can develop shots, a move that could be a game-changer for global health. This decision signifies a commitment to global vaccine equity and accessibility, especially in developing countries that have been disproportionately affected by the pandemic.
The US government’s decision to license its COVID-19 vaccine technology to the World Health Organization (WHO) is a pivotal step towards ensuring that more people worldwide have access to life-saving vaccines.
This move, driven by a desire to address the global health crisis and prevent future pandemics, will enable WHO to facilitate the production of COVID-19 vaccines in developing countries. By sharing its technology, the US aims to empower other nations to manufacture their own vaccines, ultimately contributing to a more equitable distribution of vaccines around the world.
The US License to WHO
The US government’s decision to license its COVID-19 vaccine technology to the World Health Organization (WHO) represents a significant milestone in the global fight against the pandemic. This move signifies a commitment to equitable access to life-saving vaccines, a crucial step in achieving global health security.
The U.S. licensing its key COVID-19 vaccine technology to the WHO is a major step in ensuring global access to life-saving treatments. It’s a move that will hopefully help bridge the gap in vaccine availability, especially in countries with limited resources.
This reminds me of a recent post I saw on Ask Weareteachers about a teacher struggling with job sharing. It’s a different kind of sharing, but both situations highlight the importance of collaboration and finding solutions that benefit everyone.
The more we share, the better we can address global challenges like vaccine inequity.
Impact on Global Vaccine Access and Production
This decision holds immense potential to accelerate global vaccine access and production. By granting the WHO a license, the US enables other countries to manufacture and distribute COVID-19 vaccines, particularly in low- and middle-income countries. This could dramatically increase vaccine availability, reaching more people who are currently underserved.
It’s encouraging to see the US licensing key COVID vaccine technology to the WHO, allowing other countries to develop their own shots. This global collaboration is crucial in ensuring equitable access to vaccines. Meanwhile, the news of Madison Cawthorn losing his primary after a brutal barrage of GOP attacks serves as a reminder that political battles can be fierce, even within the same party.
Ultimately, the goal should be to prioritize the health and well-being of all people, and the US sharing vaccine technology is a positive step in that direction.
Factors Influencing the US Decision
Several factors likely influenced the US government’s decision.
- The urgency to address global vaccine inequity: The stark disparity in vaccine access between wealthy and developing countries was a significant concern. The US recognized the need to bridge this gap to effectively control the pandemic globally.
- The strategic imperative of global health security: The US government likely understood that a pandemic cannot be effectively controlled in a fragmented world. By facilitating vaccine production in other countries, the US can contribute to a more robust global health security system.
The US licensing key COVID-19 vaccine technology to the WHO is a commendable step towards global health equity. However, as the world grapples with these crucial advancements, it’s important to acknowledge the looming financial burdens on many individuals. The rising cost of federal student loans, as detailed in this article its about to get more expensive to take out federal student loans heres why , adds another layer of complexity to the fight against global health disparities.
While we celebrate scientific breakthroughs, it’s crucial to address the economic realities that can hinder access to essential resources like education and healthcare.
- The potential for increased global vaccine production: By granting the WHO a license, the US is likely hoping to stimulate vaccine production in other countries, leading to a larger global supply and potentially lowering costs.
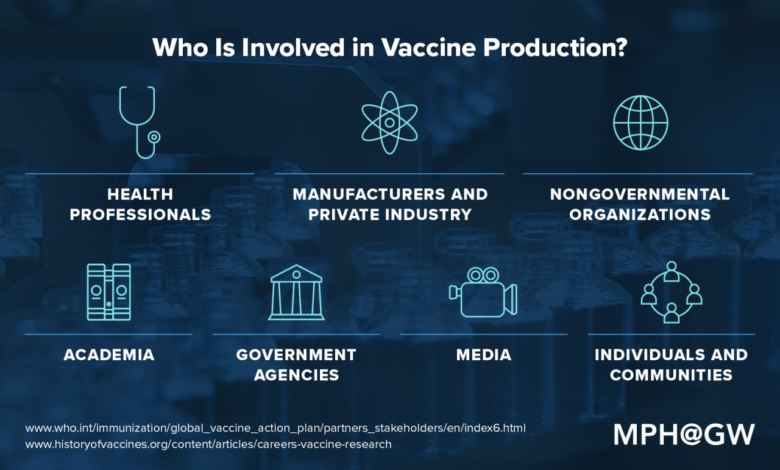
WHO’s Role in Facilitating Vaccine Production
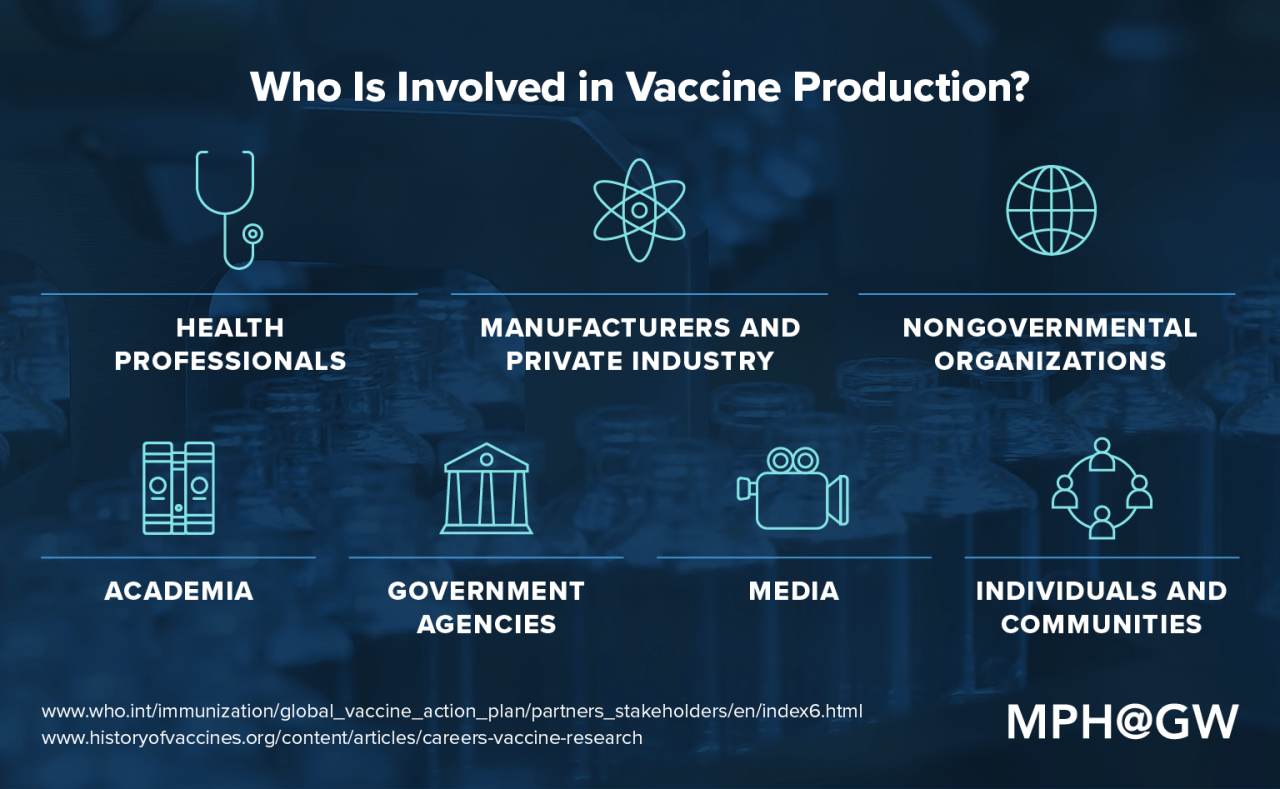
The World Health Organization (WHO) plays a crucial role in coordinating and facilitating the production of COVID-19 vaccines, particularly in developing countries. The organization’s efforts are geared towards ensuring equitable access to safe and effective vaccines for all, regardless of their geographical location or economic status.
Mechanisms for Equitable Distribution
WHO employs various mechanisms to ensure equitable distribution of vaccine technology and resources. These include:
- The COVID-19 Technology Access Pool (C-TAP):This platform facilitates the sharing of intellectual property and know-how related to COVID-19 vaccines, diagnostics, and treatments. C-TAP encourages voluntary licensing agreements between vaccine manufacturers and other companies, enabling the production of vaccines in more countries.
- The COVAX Facility:This global initiative, co-led by WHO, Gavi, and the Coalition for Epidemic Preparedness Innovations (CEPI), aims to ensure equitable access to COVID-19 vaccines for all countries. COVAX works with vaccine manufacturers to secure doses for low- and middle-income countries.
- Technical Support and Capacity Building:WHO provides technical support to developing countries to strengthen their vaccine production capacity. This includes training healthcare workers, establishing quality control systems, and supporting the development of local manufacturing facilities.
- Monitoring and Evaluation:WHO monitors the distribution and use of COVID-19 vaccines globally to ensure transparency and accountability. The organization collects data on vaccine coverage, adverse events, and vaccine effectiveness.
Impact on Global Vaccine Access and Equity: U S Licenses Key Covid Vaccine Technology To Who So Other Countries Can Develop Shots
The US decision to license its COVID-19 vaccine technology to the WHO has the potential to significantly impact vaccine access and equity in low- and middle-income countries. By enabling these countries to manufacture their own vaccines, the initiative could help address the global vaccine inequity that has characterized the pandemic response.
Potential Benefits for Global Health Security and Pandemic Preparedness, U s licenses key covid vaccine technology to who so other countries can develop shots
This initiative has the potential to bolster global health security and pandemic preparedness by:
- Increased Vaccine Production Capacity:Licensing technology to developing countries allows them to establish their own vaccine manufacturing facilities, significantly increasing global production capacity. This is particularly important for ensuring equitable access to vaccines during future pandemics.
- Reduced Dependence on High-Income Countries:By empowering developing countries to manufacture their own vaccines, the initiative reduces their dependence on high-income nations for vaccine supply. This can help to address the issue of vaccine nationalism and ensure a more equitable distribution of vaccines in future crises.
- Strengthened Health Systems:The establishment of vaccine manufacturing facilities in developing countries can contribute to the strengthening of their health systems, improving their capacity to respond to future health emergencies. This includes developing local expertise in vaccine production, quality control, and distribution.
Challenges and Considerations
While the US license holds significant promise, there are also challenges and considerations to address:
- Technological Transfer and Capacity Building:Transferring complex vaccine technology to developing countries requires significant investment in training, infrastructure, and technical expertise. This process needs to be carefully managed to ensure successful implementation and avoid potential delays.
- Intellectual Property Rights:Balancing intellectual property rights with the need for equitable vaccine access is a complex issue. The US license needs to be structured in a way that encourages technology transfer while protecting the interests of the original developers.
- Financing and Sustainability:Setting up and maintaining vaccine manufacturing facilities in developing countries requires significant financial resources. Sustainable funding models need to be developed to ensure the long-term viability of these facilities.
Final Thoughts
The US decision to license its COVID-19 vaccine technology to WHO marks a significant milestone in global health. This move, driven by a commitment to global vaccine equity, will hopefully pave the way for increased vaccine production and access in developing countries.
While there are challenges to overcome, this initiative holds immense potential to improve global health security and pandemic preparedness, ensuring that future pandemics can be addressed more effectively.